
14/02/2024 - Covid-19
It is a monoclonal antibody with prophylactic and therapeutic action, isolated from blood samples of a patient infected by SARS-CoV-2 during the first wave of the pandemic.
The antibody has been designed and developed by researchers from the Hospital del Mar Research Institute, the IrsiCaixa AIDS Research Institute, the National Center for Biotechnology and the Center for Genomic Regulation. In addition, this new treatment has been patented pending commercial development.
This new treatment has been patented pending commercial development. The results of the work have been published in the journal Nature Communications.
A study by the Hospital del Mar Research Institute, the IrsiCaixa AIDS Research Institute, a center jointly promoted by the "la Caixa" Foundation and the Department of Health of the Generalitat de Catalunya, the Centro Nacional de Biotecnología, which belongs to the Spanish National Research Council (CNB-CSIC), and the Protein Technologies Unit of the Center for Genomic Regulation (CRG) has made it possible to develop a new antibody that is active against all existing variants of SARS-CoV-2, including the currently circulating subvariants of omicron. It is a monoclonal antibody - an immune system protein developed in the laboratory - called 17T2. The work, in which a scientific team from CIBER Infectious Diseases (CIBERINFEC) has also participated, has just been published in the journal Nature Communications.
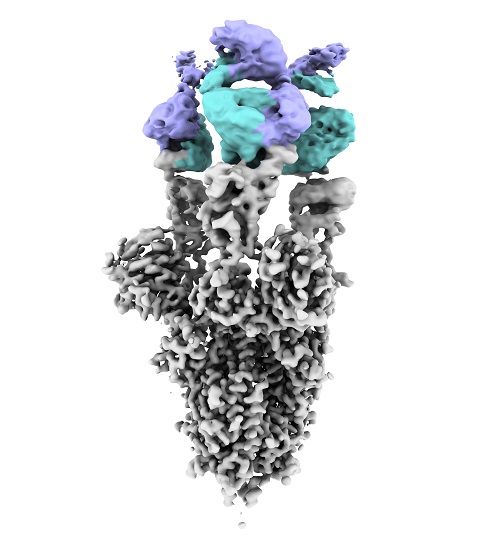
Image obtained by electron cryomicroscopy of the SARS-CoV2 virus Spike protein (in gray) with the new antibody bound (heavy chain in blue and light chain in violet). Andrea Modrego, CNB-CSIC
The isolation of the new antibody was possible thanks to blood samples from a patient infected by SARS-CoV-2 in March 2020, during the first wave of the pandemic. From these samples, some B lymphocytes, the blood cells responsible for producing antibodies, were selected. Specifically, those that generated specific antibodies against the spicule protein, which allows the virus to infect human cells, multiply and trigger COVID-19, were chosen.

Dr. Giuliana Magri.
Julià Blanco, co-leader of the study and IGTP researcher at IrsiCaixa.
Using genetic engineering techniques, the researchers reproduced these antibodies in the laboratory. Once this had been achieved, they evaluated in vitro their neutralizing activity - that is, their ability to bind to the virus and block it - against the different SARS-CoV-2 variants existing to date. Thus, they were able to select the antibody that was able to neutralize all of them, including XBB.1.16 and BA.2.86, from which the most worrying variants are currently derived. As Dr. Giuliana Magri, leader of the study, who was a researcher at the Hospital del Mar Research Institute during the study, points out, “our antibody maintains neutralizing activity against all variants of SARS-CoV-2". In turn, Dr. Benjamin Trinité, one of the first authors of the study and senior researcher at IrsiCaixa, highlights the importance of the finding and mentions that "the latest variants of the virus have incorporated dozens of mutations that make the work of previously developed antibodies difficult, since they cannot bind as effectively. Having a treatment that is effective even if new variants of SARS-CoV-2 appear can change the rules of the game when it comes to fighting the infection".
The study analyzed in a mouse model the therapeutic capacity of the antibody, but also the prophylactic, i.e. preventive, activity of the new treatment, certifying its ability to significantly reduce lung lesions and viral load. In this sense, Dr. Magri emphasizes that the study "demonstrates that the antibody developed shows prophylactic and not only therapeutic activity, which identifies it as a potential candidate for clinical preventive interventions and treatment of the infection".
Finally, the team carried out a detailed analysis of the structure of the antibody bound to the spicule protein, in order to understand how it works and how it manages to maintain neutralizing activity, despite the mutations accumulated by the SARS-CoV-2 virus. This structural study, carried out at CNB-CSIC by the team of Dr. Rocío Arranz, co-leader of the study, allows us to affirm that "this antibody has the ability to bind to a large area of the virus spicule, which confers the ability to neutralize all variants and prevent new mutations from evading this neutralization. This suggests that, in this area of interaction, there is a conserved region in the spicule, which could be essential for the ability of the virus to infect human cells."
Before its development for use in patients, it will be necessary to conduct a clinical trial in humans. For the time being, there is an active European patent associated with this project.
"Having antibodies such as 17T2 is key to being able to protect immunocompromised individuals at high risk of developing severe COVID-19. The results obtained show that it is possible to design tools capable of blocking all variants of the same virus. In fact, this opens the way to the design of antibodies and/or pan-coronavirus vaccines, that is, with the capacity to combat different types of coronavirus", concludes Dr. Julià Blanco, co-leader of the study and IGTP researcher at IrsiCaixa.
This research project has been supported by grants from the COVID-19 call of the Generalitat de Catalunya, as well as from the Miguel Servet research program, and has been partially funded by the #YoMeCorono sponsorship campaign and the Fundació Glòria Soler.
de Campos-Mata, L., Trinité, B., Modrego, A. et al. A monoclonal antibody targeting a large surface of the receptor binding motif shows pan-neutralizing SARS-CoV-2 activity. Nat Commun 15, 1051 (2024). https://doi.org/10.1038/s41467-024-45171-9
© Institut Hospital del Mar
d'Investigacions MèdiquesLegal Notice and Privacy Policy | Cookie Policy | Site Index | Accessibility | Find Us | Contact