
This project is focussed to study how extracellular Wnt proteins control epithelial tumor cell invasion. Wnt factors regulate different cellular processes during development and disease, particularly in cancer. Wnt ligands interact with transmembrane Frizzled (Fz) receptors and different co-receptors, depending on which they stimulate canonical or non-canonical Wnt signalling pathways. The canonical Wnt3a binds to Fz and co-receptor LRP5/6 promoting β-catenin stabilization and transcriptional activation of β-catenin-dependent genes. In contrast, non-canonical Wnt5a ligand binds to Fz and co-receptor Ror2 and produces a decrease in β-catenin through the activation of the E3 ligase Siah2. Remarkably, although the effects on β-catenin levels are contrary, both Wnt5a and Wnt3a stimulate the invasion of tumor cells.
When searching common elements to canonical and non-canonical Wnts, we have recently observed that both stimulate a new branch involving Fz2, Src, Fyn and Stat3, required for transcription of canonical and non-canonical Wnt targets involved in cell invasion (see Figure 1). Both types of Wnt ligands stimulate Fz2 tyrosine phosphorylation, Fyn binding to Fz2, Fyn activation and Fyn-dependent Stat3 phosphorylation. Canonical Wnt3a and non-canonical Wnt5a require Src for Fz2 tyrosine phosphorylation; Src binds to canonical and non-canonical co-receptors (LRP5/6 and Ror2, respectively) and is activated both, by Wnt3a and Wnt5a. Remarkably, this new Fz2/Fyn/Stat3 branch is incompatible with the classical Fz2/Dishevelled/Axin pathway. However, both routes are required for the activation of tumor cell invasion by Wnt. Therefore, our results extend the knowledge of canonical Wnt signalling demonstrating additional roles for Fyn in this pathway and characterize a new route activated by all Wnts and required for cell invasion.
For both canonical and non-canonical Wnt pathways, Stat3 activation is required for transcription of epithelial-to-mesenchymal-transition-associated genes, such as Snail1 (Villarroel A et al., 2020), that controls tumor cell invasion and chemo-resistance. When analysing the relative contribution of canonical and non-canonical Wnt to Snail1 expression we have determined that it is mainly dependent on non-canonical Wnt; therefore, chemo-resistance in tumor cells with high Snail1 expression is alleviated by blocking the Ror2 activity. We are working in other signals controlled by non-canonical Wnts in tumor cells.
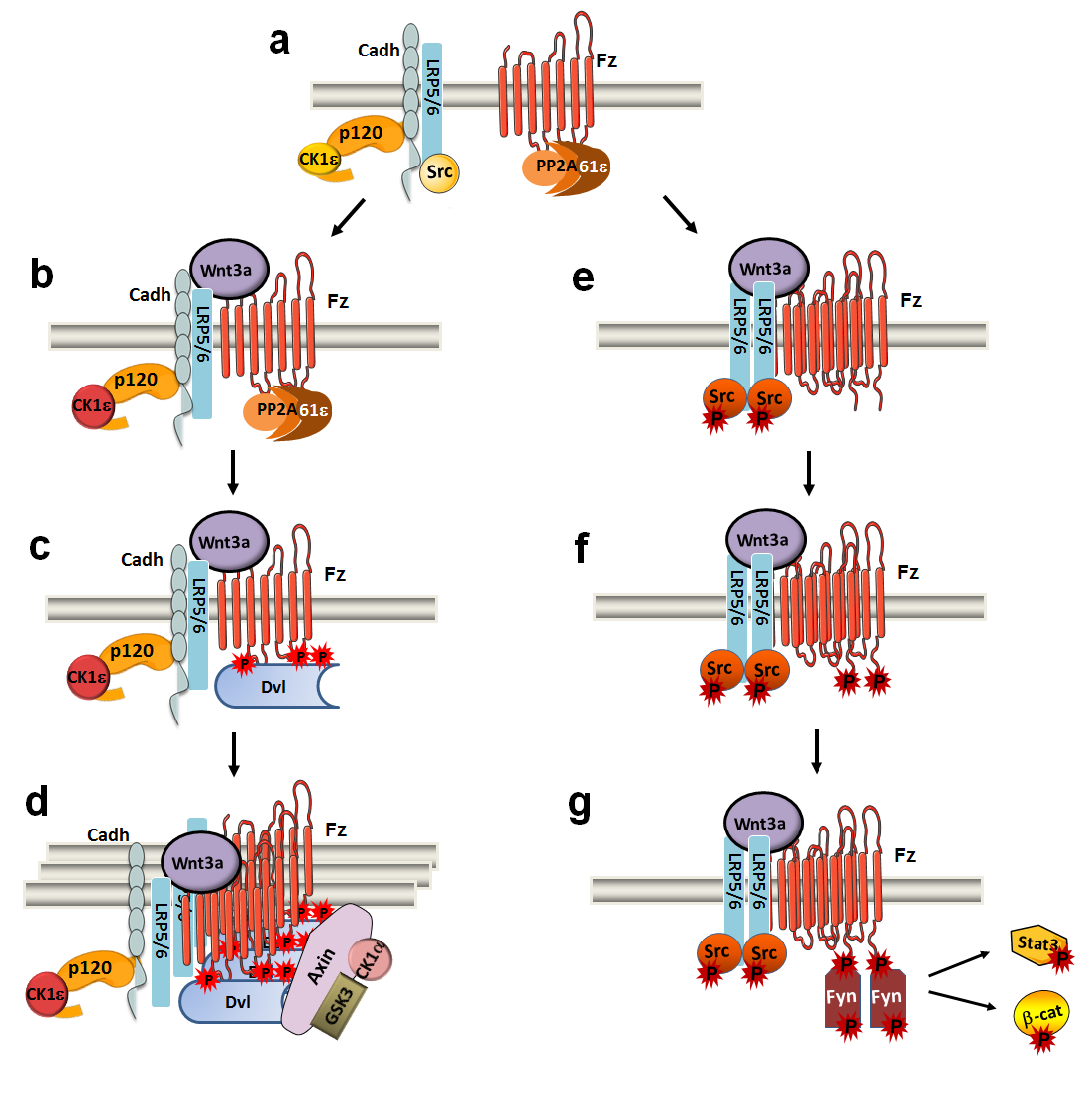
Figure 1. Canonical Wnt ligands induce two mutually exclusive Dvl2- and Fyn-dependent arms. (a) In Wnt OFF, LRP5/6 co-receptor interacts through p120-catenin and E-cadherin with inactive CK1ε and Src (both inactive kinases are shown in yellow). (b) Wnt3a promotes that PP2A phosphatase, associated to Fz2 through the PR61ε regulatory subunit, moves closer to CK1ε, and dephosphorylates and activates CK1ε (in orange). (c) CK1ε increases Dvl2 phosphorylation and its binding to Fz2. (d) Dvl2′s association leads to signalosome assembly, axin recruitment, and further responses of this pathway. (e) LRP5/6 dimerization promotes Src activation and (f) Src-dependent phosphorylation of Tyr552 in Fz2. (g) Phospho-Tyr552 Fz2 binds and activates Fyn, promoting the phosphorylation of Stat3. Fyn also phosphorylates β-catenin Tyr142, releasing β-catenin from α-catenin and cadherin and thereby facilitating its transcriptional activity. From Villarroel et al, 2020.
Four selected recent publications:
© Institut Hospital del Mar
d'Investigacions MèdiquesLegal Notice and Privacy Policy | Cookie Policy | Site Index | Accessibility | Find Us | Contact